[ad_1]
The Supreme Court docket is about to listen to oral arguments on Tuesday, March 26, in a case that might have a major impression on how mifepristone — the primary drug utilized in a two-drug remedy abortion routine — is used and prescribed in america.
The case, FDA v. Alliance for Hippocratic Medication, surrounds the U.S. Meals and Drug Administration’s (FDA) rolling again of security restrictions for mifepristone, together with its 2016 motion extending the permissible gestational age of the newborn for which a lady or lady might take abortion medicine from seven weeks gestation to 10 weeks gestation and its 2021 rule change permitting abortionists to ship mifepristone by way of the mail. The FDA additionally not too long ago made everlasting its rule to permit girls and women to obtain a prescription for mifepristone by way of telemedicine.
The Alliance Defending Freedom (ADF) filed the lawsuit in November of 2022 in opposition to the FDA on behalf of 4 nationwide medical associations and a number of other docs, alleging the company “selected politics over science and authorised chemical abortion medicine to be used in america.”
“The U.S. Meals and Drug Administration (FDA) — the federal company accountable for guaranteeing the security of medication that Individuals take — has betrayed girls and women,” Erik Baptist, senior counsel with the ADF and co-counsel on the case, stated throughout a press convention in Washington, DC, on Thursday.
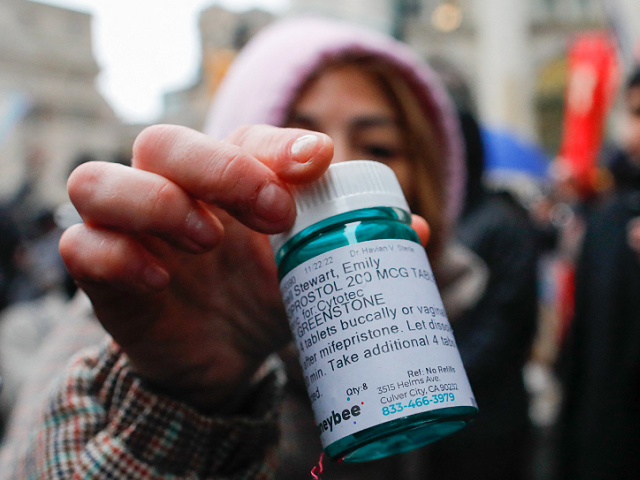
A professional-abortion activist shows abortion drugs as she counterprotests throughout an anti-abortion demonstration on March 25, 2023, in New York Metropolis. (KENA BETANCUR/AFP by way of Getty Photos)
“Ladies ought to have the continuing care of a health care provider when taking excessive danger medicine. However in recent times, the FDA has recklessly eliminated almost each safeguard the company beforehand thought-about vital for abortion medicine, together with in-person physician visits to test for ectopic pregnancies, extreme bleeding, and life-threatening infections,” Baptist added. “With out query, the FDA’s actions made taking high-risk abortion medicine much less protected for ladies.”
Justices agreed to take up the case in December of 2023. In September, each President Joe Biden’s Division of Justice (DOJ) and Danco Laboratories LLC, which distributes mifepristone underneath the model identify Mifeprex, requested the Supreme Court docket to reverse a decrease courtroom resolution halting two FDA actions that loosened restrictions on the abortion tablet. The Supreme Court docket in the end granted a listening to to each instances, consolidating them into one with a complete of 1 hour allotted for oral argument.
Biden’s DOJ and Danco Laboratories appealed an August ruling from the U.S. Court docket of Fifth Circuit, which discovered that the FDA’s 2016 resolution to permit the abortion tablet to be taken as much as ten weeks of being pregnant as a substitute of seven weeks is illegal. The courtroom stated the identical of the FDA’s 2021 rule change, which allowed the abortion tablet to be mailed on to sufferers and allowed medical professionals apart from docs to prescribe mifepristone.
“In loosening mifepristone’s security restrictions, FDA failed to deal with a number of vital issues about whether or not the drug can be protected for the ladies who use it,” reads the panel opinion, penned by Decide Jennifer Walker Elrod.
“It failed to contemplate the cumulative impact of eradicating a number of vital safeguards on the similar time. It failed to contemplate whether or not these ‘main’ and ‘interrelated’ modifications may alter the danger profile, such that the company ought to proceed to mandate reporting of non-fatal opposed occasions,” the opinion continued. “And it failed to collect proof that affirmatively confirmed that mifepristone may very well be used safely with out being prescribed and allotted in particular person.”
Elrod wrote that on the preliminary stage, plaintiffs “have made a considerable exhibiting” that the 2016 and 2021 rule modifications violate the Administrative Process Act (APA).
Regardless of the Fifth Circuit’s ruling in opposition to the federal government and the drug producer, mifepristone has remained accessible underneath present laws whereas the litigation continues. The Supreme Court docket preemptively paused any ruling from an appeals courtroom this spring, pending a petition for the Supreme Court docket to take the case.
The ADF’s lawsuit factors to 6 discrete company actions because the legalization of mifepristone and misoprostol in 2000. The ADF alleges that the company was solely capable of approve the drug by falsely classifying being pregnant as an “sickness.” The lawsuit additionally alleges that the FDA by no means studied the security of mifepristone underneath the labeled situations of use, ignored the potential impacts of the hormone-blocking routine on the growing our bodies of adolescent women, disregarded proof that chemical abortion medicine trigger extra problems than surgical abortion, and eradicated vital safeguards for pregnant women and girls who take the routine.
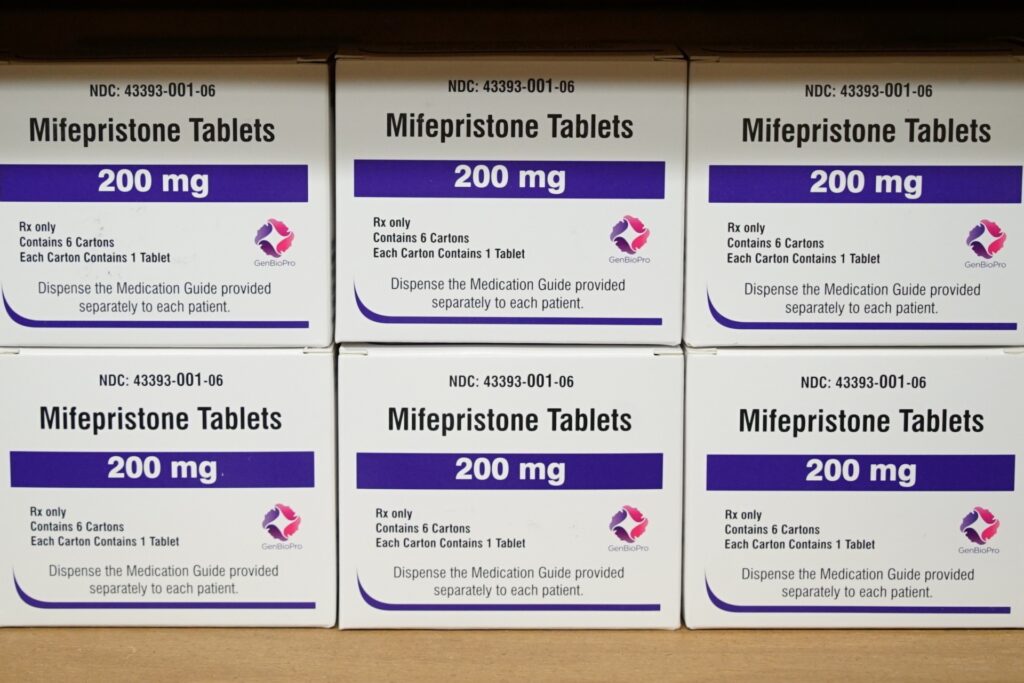
Containers of the drug mifepristone sit on a shelf on the West Alabama Ladies’s Middle in Tuscaloosa, AL, on March 16, 2022. (AP Photograph/Allen G. Breed, File)
The lawsuit particulars how, in 2016, the FDA prolonged the permissible gestational age for which a lady or lady might take the abortion medicine. Then, in 2021, the FDA allowed abortionists to ship mifepristone by way of the mail, which the ADF says was “in direct violation of federal regulation.”
The grievance alleges:
The entire FDA’s actions on chemical abortion medicine — the 2000 approval, the 2016 main modifications, the 2019 generic drug approval, and the 2 2021 actions to remove the in-person allotting requirement — didn’t acknowledge and tackle the federal legal guidelines that prohibit the distribution of chemical abortion medicine by postal mail, specific firm, or frequent provider. As a substitute, the FDA’s actions permitted and generally even inspired these unlawful actions.
The Fifth Circuit took up the case after U.S. District Decide Matthew Kacsmaryk of the U.S. District Court docket for the Northern District of Texas handed down a 67-page resolution that the FDA’s choices had been unlawful underneath federal regulation, issuing a nationwide injunction blocking the abortion tablet.
Days later, the U.S. Court docket of Appeals for the Fifth Circuit partially granted a keep requested by the Biden Justice Division. The courtroom’s 42-page opinion briefly placed on maintain the a part of the choice concerning the 2000 FDA resolution as a result of it could be previous the deadline for bringing authorized challenges, although added that it was a “shut name” and that the courtroom may go the other way after receiving further authorized arguments. However the appellate courtroom rejected a keep on something from 2016 to the current, affirming the trial courtroom’s injunction.
The Supreme Court docket then put a maintain on Kacsmaryk’s total resolution. As Breitbart Information beforehand famous, an administrative keep shouldn’t be in any method a mirrored image on the authorized deserves of the case. A Supreme Court docket justice can challenge one simply to protect the established order whereas the courtroom is receiving arguments from either side.

Three members of the Ladies’s March group protest in help of entry to abortion remedy exterior the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. (AP Photograph/David Erickson)
The Supreme Court docket declined to take up an enchantment from the ADF asking justices to rule on the legality of the FDA’s 2000 approval of mifepristone.
In a medicine abortion, mifepristone blocks the motion of progesterone, which the mom’s physique produces to nourish the being pregnant. When progesterone is blocked, the liner of the mom’s uterus deteriorates, and blood and nourishment are minimize off to the growing child, who then dies contained in the mom’s womb. The drug misoprostol (additionally known as Cytotec) then causes contractions and bleeding to expel the newborn from the mom’s uterus.
This week. the pro-abortion Guttmacher Institute launched a report which discovered that remedy abortions accounted for 63 p.c of all abortions throughout the formal U.S. well being care system in 2023 after the Supreme Court docket overturned Roe v. Wade — which means an estimated 642,700 unborn infants died in remedy abortions. The share is up from an estimated 53 p.c in 2020 and 39 p.c in 2017. The report didn’t account for abortion drugs obtained by way of underground nationwide and worldwide networks, together with people who ship drugs to girls in pink states.
The Biden administration has relentlessly promoted remedy abortions, and in January of 2023, the FDA allowed retail pharmacies to dispense abortion drugs. On the similar time, a number of Democrat governors have stockpiled abortion drugs whereas enacting defend legal guidelines that shield well being care suppliers who prescribe mifepristone to girls and women in states with legal guidelines defending the unborn.
The case is FDA v. Alliance for Hippocratic Medication, No. 23-235 within the Supreme Court docket of america.
Katherine Hamilton is a political reporter for Breitbart Information. You possibly can comply with her on X @thekat_hamilton.
[ad_2]


